Why in News?
- USA's Victoria Gray is the world’s first sickle cell anaemia (SCA) patient to recover with the revolutionary gene-editing therapy.
- Gray underwent a clinical trial in 2017 for the drug Casgevy, which uses the innovative gene-editing tool CRISPR-Cas9 (Clustered Regularly Interspaced Short Palindromic Repeats and associated protein 9).
What’s in Today’s Article?
- What is Sickle Cell Anaemia (SCA)?
- How the Pathbreaking Gene Therapy for SCA Works?
- Significance of this Pathbreaking Gene Therapy for SCA for India
- Concerns Regarding the Gene Therapy for SCA and Way Ahead
What is Sickle Cell Anaemia (SCA)?
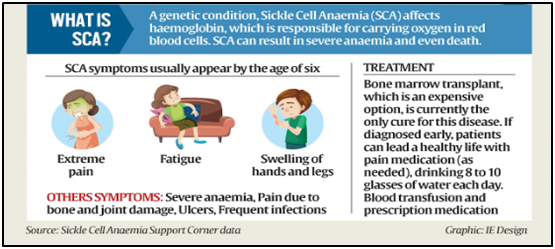
- A genetic disease, SCA arises from mutations in haemoglobin-carrying genes, causing red blood cells to assume a crescent shape.
- This potentially obstructs blood flow and leads to severe pain, organ damage, strokes, and other complications.
- Globally, the current remedy for the disease is limited to bone marrow transplants (which must come from a closely matched donor and carries a risk of rejection).
How the Pathbreaking Gene Therapy for SCA Works?
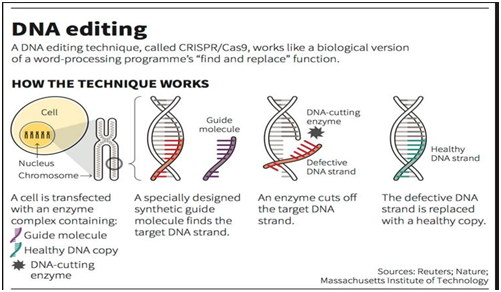
- The CRISPR-Cas9 technique basically involves modifying the patient’s DNA, specifically targeting and replacing the faulty haemoglobin gene with a healthy one.
- To do this, stem cells are taken out of the bone marrow, edited in a laboratory and then infused back into the patient.
- This restores normal haemoglobin function, offering a potential cure for a lifetime.
- About 29 patients were administered the treatment (along with Gray), of whom 28 have no pain and will be followed up for lasting cure.
- The therapy also works for patients suffering from transfusion-dependent ß-thalassemia.
- Recently, the UK approved (became first country to do so)the therapy - called Casgevy, as it has been the only permanent, innovative and first-of-its-kind gene-editing treatment option.
Significance of this Pathbreaking Gene Therapy for SCA for India:
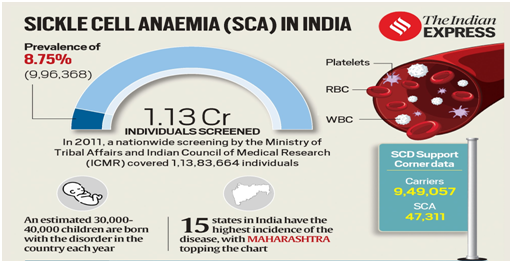
- The breakthrough is of particular significance to India, which has the second-highest disease burden of SCA globally after African countries.
- An estimated 30,000-40,000 children in India are born with the disorder every year.
- In 2019, a nationwide screening by the Ministry of Tribal Affairs and the Indian Council of Medical Research (ICMR) found the disease to be prevalent in 8.75% of those screened.
- Latest data shows that one in 86 births among the Scheduled Tribe (ST) population is affected by SCA, with higher rates in central, western and southern India.
- In her 2023-24 Budget presentation, the Finance Minister of India had said that the government aimed to eliminate the disease by 2047.
Concerns Regarding the Gene Therapy for SCA and Way Ahead:
- While Vertex and CRISPR Therapeutics (the manufacturers) have yet to set a price for the gene-editing therapy in the UK, at the moment it is expected to exceed Rs 1 crore in India, which could potentially limit access.
- However, government intervention and subsidies, along with crowdfunding and philanthropy, can enable access to cure.
- The UK’s approval would inspire Indian researchers to develop their own innovative therapies of CRISPR like they did with CAR-T for blood cancer, ultimately making the treatment more affordable.









