In News:
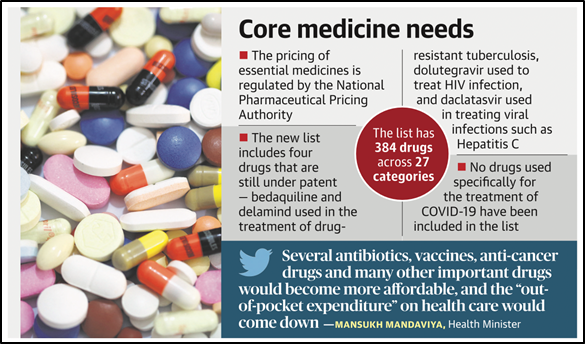
- With the addition of 34 new drugs, the National List of Essential Medicines (NLEM) has a total of 384 listed as essential medicines.
What’s in today’s article:
- About NLEM (Meaning, Purpose, Issuing body, etc.)
- News Summary (About NPPA)
National List of Essential Medicines (NLEM):
- Essential medicines are those that satisfy the priority healthcare needs of the majority of the population.
- The primary purpose of NLEM is to promote rational use of medicines considering the three important aspects i.e., cost, safety and efficacy.
- The medicines in NLEM should be available at affordable costs and with assured quality.
- The first National List of Essential Medicines of India was prepared and released in 1996.
- Under the latest NLEM 2015, a total 376 drugs are under price control.
- The revision in the NLEM was delayed in 2020 due to the Covid-19 pandemic.
- The drugs in the NLEM are included in the schedule category and their price is regulated by the National Pharmaceutical Pricing Authority (NPPA).
- Issued by: Ministry of Health and Family Welfare
- It is based on World Health Organisation’s (WHO) Model List of Essential Drugs.
News Summary:
- 384 drugs have been included in this list with addition of 34 drugs, while 26 from the previous list have been dropped.
- Several antibiotics, vaccines and anti-cancer drugs are set to become more affordable with their addition to the list.
- Inclusion of a drug in the NLEM mandates that it is sold at prices fixed by the NPPA.
About National Pharmaceuticals Pricing Authority (NPPA):
- National Pharmaceutical Pricing Authority (NPPA) was constituted by a resolution of the Government of India in 1997.
- It is an attached office of the Department of Pharmaceuticals (DoP), Ministry of Chemicals & Fertilizers.
- It acts as an independent Regulator for pricing of drugs and to ensure availability and accessibility of medicines at affordable prices.
Functions:
- To implement and enforce the provisions of the Drugs (Prices Control) Order in accordance with the powers delegated to it.
- To deal with all legal matters arising out of the decisions of the Authority.
- To monitor the availability of drugs, identify shortages, if any, and to take remedial steps.
- To collect/maintain data on production, exports and imports, market share of individual companies, profitability of companies etc, for bulk drugs and formulations.
- To undertake and/or sponsor relevant studies in respect of pricing of drugs/ pharmaceuticals.
- To render advice to the Central Government on changes/revisions in the drug policy.
- To render assistance to the Central Government in the parliamentary matters relating to the drug pricing.










