Why in News?
Novo Nordisk, the Danish pharmaceutical giant behind the popular drugs Wegovy (for weight loss) and Ozempic (to treat type 2 diabetes in adults), has requested that the U.S. Food and Drug Administration (FDA) stops the compounding of semaglutide-based medications.
The company argues that compounded versions, created to meet rising demand, may pose safety risks to patients.
What’s in Today’s Article?
- Comparing Generic and Compounded Drugs
- Key Issues in Drug Compounding
- Proposed Solution to Address Issues with Drug Compounding
Comparing Generic and Compounded Drugs:
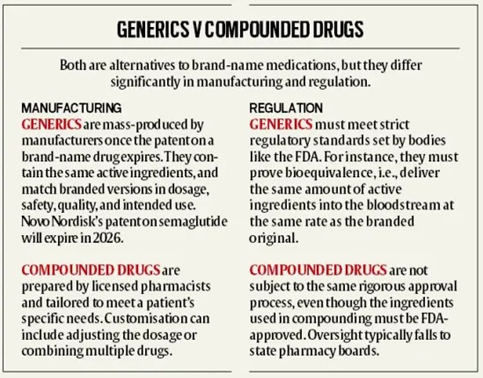
Key Issues in Drug Compounding:
- Limited regulations:
- Under FDA guidelines, licensed pharmacists can legally compound medications to meet patient needs, particularly when branded versions are unavailable.
- With Wegovy and Ozempic in high demand, compounding pharmacies have been formulating their own versions, prompting Novo Nordisk’s intervention.
- American pharmaceutical firm Eli Lilly similarly sought to halt compounded versions of its drugs Mounjaro and Zepbound, intended for diabetes and obesity treatment.
- The FDA has yet to issue a decision on either case.
- Purity risks: Semaglutide’s intricate structure is challenging to replicate accurately. Hence, the compounded versions may lack precision, potentially compromising purity and stability.
- Risk of incorrect dosing:
- The FDA-approved semaglutide is delivered through a single-use pen injector, ensuring precise dosage and clear usage instructions.
- Compounded drugs, however, are often dispensed in multi-dose vials or syringes, raising the risk of incorrect dosing.
- Reports cite patients accidentally overdosing, resulting in severe side effects like nausea and vomiting.
- Risking severe health issues:
- Bioavailability - the degree to which the drug reaches the bloodstream - is crucial for semaglutide.
- Without proper absorption, compounded versions may fail to provide the intended treatment effects, risking severe health issues like heart disease, nerve damage, and kidney complications.
- Contamination risks:
- Compounded semaglutide requires sterile facilities and precise handling to avoid contamination.
- In recent years, the FDA has flagged sterility issues at compounding pharmacies, leading to significant recalls.
Proposed Solution to Address Issues with Drug Compounding:
- Adding Semaglutide to DDC list:
- Novo Nordisk petitioned the FDA to place semaglutide on the Demonstrable Difficulties for Compounding (DDC) list, which would restrict its compounding when commercial options are available.
- The FDA assesses drugs for the DDC list based on stability, bioavailability, dosage requirements, and sterility demands, all factors Novo Nordisk highlights in its case.









