Why in news?
- Recently, the Supreme Court came down heavily on Baba Ramdev’ Patanjali Ayurved for publishing misleading advertisements.
- The apex court banned it from marketing its products until further orders are passed.
What’s in today’s article?
- Consumer Protection Act
- Allegations against Patanjali
- Legal argument against Patanjali’s actions
Consumer Protection Act, 2019?
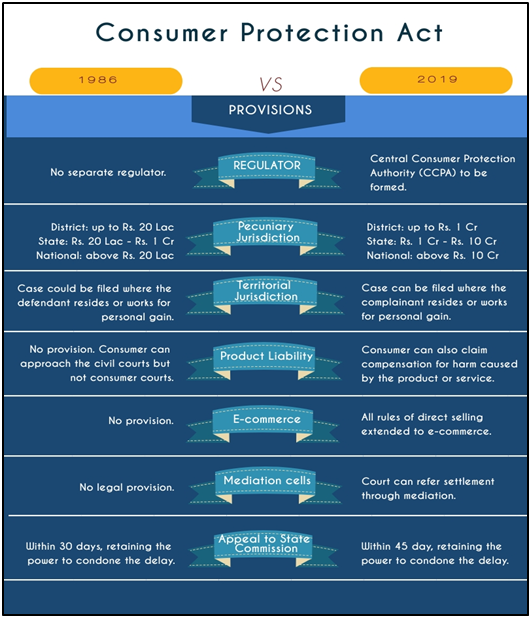
- The Consumer Protection Act, 2019 replaced the Consumer Protection Act, 1986, and seeks to widen its scope in addressing consumer concerns.
- The new Act recognises offences such as providing false information regarding the quality or quantity of a good or service, and misleading advertisements.
- It also specifies action to be taken if goods and services are found “dangerous, hazardous or unsafe”.
- The Act came into force in July 2020 and it will empower consumers and help them in protecting their rights through its various notified rules and provisions.
What is Central Consumer Protection Authority (CCPA)?
- About: The CCPA is a statutory body constituted under Section 10 of the Consumer Protection Act, 2019.
- Mandate: To protect the rights of the consumer by cracking down on unfair trade practices, and false and misleading advertisements that are detrimental to the interests of the public and consumers.
- Concerned Ministry: Ministry of Consumer Affairs, Food and Public Distribution
- Powers & Functions of CCPA: It is empowered to:
- conduct investigations into violation of consumer rights and institute complaints / prosecution,
- order recall of unsafe goods and services,
- order discontinuation of unfair trade practices and misleading advertisements,
- impose penalties on manufacturers/endorsers/publishers of misleading advertisements.
Allegations against Patanjali
- In 2022, Patanjali published an advertisement titled “MISCONCEPTIONS SPREAD BY ALLOPATHY: SAVE YOURSELF AND THE COUNTRY FROM THE MISCONCEPTIONS SPREAD BY PHARMA AND MEDICAL INDUSTRY.”
- After this, the Indian Medical Association (IMA) filed a petition at the apex court.
- The petition details other instances where Baba Ramdev called allopathy a stupid and bankrupt science, and made claims about allopathic medicine being responsible for Covid-19 deaths.
- The IMA also accused Patanjali of contributing to vaccine-hesitancy during the pandemic by spreading false rumours.
- The IMA claims that these attacks against modern medicine come alongside Patanjali’s own efforts to make false and unfounded claims about curing certain diseases through the use of Patanjali products.
Legal argument against Patanjali’s actions
- The IMA claimed that the advertisement was in direct violation of the Drugs & Other Magical Remedies Act, 1954 (DOMA), and the Consumer Protection Act, 2019 (CPA).
- The publishing of false and misleading advertisements is an offence under both statutes.
- Section 4 of the DOMA
- Under Section 4 of the DOMA, there is a prohibition against publishing misleading advertisements relating to a drug.
- Publishing a misleading advertisement under the DOMA is punishable with up to six months imprisonment, and/or a fine for the first offence.
- On the second offence, the period of imprisonment can extend to one year.
- Consumer Protection Act, 2019
- Section 2(28) of the Consumer Protection Act, 2019 deals with the ‘misleading advertisement’.
- Section 89 of the CPA contains more stringent punishments for false or misleading advertisements.
- First time violations may invite penalties up to Rs 10 lakh and imprisonment for a term which may extend to two years.
- Subsequent violations may attract penalties up to Rs 50 lakh and imprisonment for a term which may extend to five years.
- The CPA also provides the definition for a misleading advertisement.
- It includes advertisements which:
- give a false description of the product or service, partakes in unfair trade practices,
- deliberately conceals important information, or is likely to mislead the consumer about the nature, substance, quantity or quality of the product or service.
- Violation of MoU signed by the Ministry of AYUSH and the Advertising Standards Council of India
- The IMA has also highlighted the MoU signed by the Ministry of AYUSH and the Advertising Standards Council of India in January 2017.
- AYUSH agreed to identify misleading advertisements that may be in violation of the DOMA, and send complaints to the Council for review.









