Why in news?
- Uzbekistan has claimed that at least 18 children in the country have died after allegedly taking an India-manufactured cough syrup.
- The health ministry of Uzbekistan said that the children who died had consumed cough syrup Dok-1 Max - manufactured by Noida-based Marion Biotech.
- After the incident in Gambia, the current incident may harm the India’s reputation as the pharmacy of the world.
What’s in today’s article:
- The pharmaceutical industry in India
- News Summary
The pharmaceutical industry in India
Notable achievements
- The Indian Pharmaceuticals industry plays a prominent role in the global pharmaceuticals industry.
- India ranks 3rd worldwide for production by volume and 14th by value.
- India is the largest provider of generic medicines globally, occupying a 20% share in global supply by volume.
- The pharmaceutical industry in India offers 60,000 generic brands across 60 therapeutic categories.
- It is the leading vaccine manufacturer globally. 60% of the world’s vaccines comes from India.
Industry scenario
- Foreign Direct Investment (FDI)
- 100% FDI in the Pharmaceutical sector is allowed under the automatic route for greenfield pharmaceuticals.
- 100% FDI in the pharmaceutical sector is allowed in brownfield pharmaceuticals; wherein 74% is allowed under the automatic route and thereafter through the government approval route.
- Market Size
- The pharmaceutical industry in India is currently valued at $50 bn. It is expected to reach $65 bn by 2024 and to $120 bn by 2030.
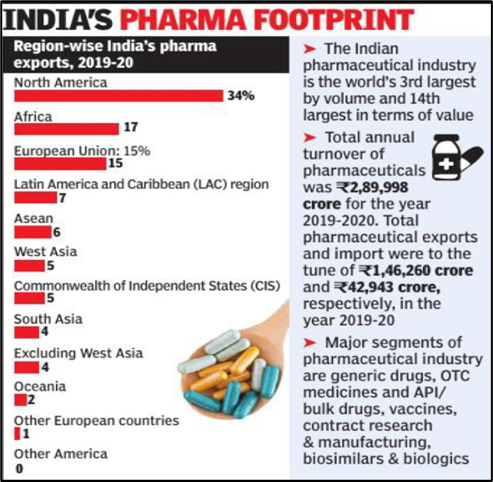
- Export
- India is a major exporter of Pharmaceuticals, with over 200+ countries served by Indian pharma exports.
- India supplies over 50% of Africa’s requirement for generics, ~40% of generic demand in the US and ~25% of all medicine in the UK.
- For the period 2021-22, export of drugs and pharma products stood at $24.6 bn compared to $24.44 bn as of 2020-21.
- The Indian pharma industry witnessed exponential growth of 103% during 2014-22 from $11.6 bn to $24.6 bn.
- Support by the govt
- The Indian pharmaceuticals market is supported by the following Production Linked Incentive (PLI) Schemes.
- PLIs are aimed to boost domestic manufacturing capacity, including high-value products across the global supply chain.
News Summary
- India has launched an inquiry into the alleged role of cold and flu syrup manufactured by Noida-based Marion Biotech, in the recent deaths of 18 children in Uzbekistan.
- The Uzbek health ministry alleged that the kids died after drinking Dok1 Max and it contains unacceptable amounts of Ethylene Glycol (EG).
- The Uzbekistan tragedy comes weeks after a similar incident was reported from Gambia.
Tragedy in Gambia
- It was alleged that 69 children died in the African nation after consuming cough syrup exported by an Indian firm.
- The World Health Organisation (WHO) issued a medical product alert in the matter stating that samples of the cough syrup had been found to contain unacceptable amounts of diethylene glycol and ethylene glycol as contaminants.
- India, however, slammed the WHO saying the deduction that India-made cough syrup was responsible for the death of children in Gambia was premature.
India’s image might take a hit
- Experts believe that repeated reports of such incidents may harm the country’s reputation as the pharmacy of the world.
- Therefore, renewed efforts are being made by the government to strengthen regulatory mechanisms for drug manufacturers.
- Recently, the government said it had prepared an action plan for nationwide inspection of manufacturing units which are identified to be at the risk of manufacturing Not of Standard Quality (NSQ)/adulterated/spurious drugs.










