Key Scientific Findings:
- A novel heterostructure, combining Copper Tungsten Oxide (CuWO₄) and Copper Oxide (CuO), has been created to exploit the Built-In Electric Field (BIEF) effect for enhanced hydrogen evolution.
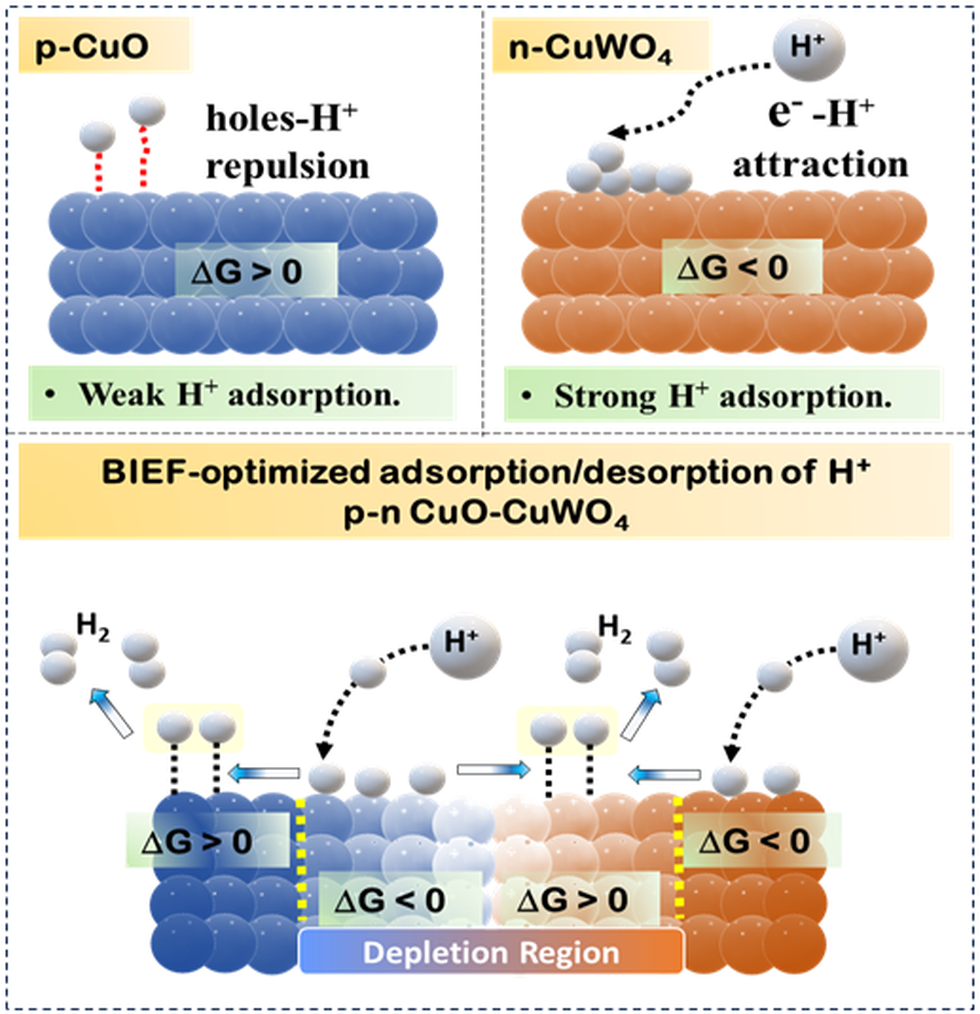
- The structure is formed by growing CuWO₄ nanoparticles over a Cu(OH)₂ precursor, leading to a p-n heterojunction that creates an asymmetric electronic environment.
- This BIEF plays a crucial role in modulating proton adsorption and desorption, directly influencing the Hydrogen Evolution Reaction (HER)
Mechanism of Proton Adsorption
- The interface between CuO and CuWO₄ shows variation in Gibbs Free Energy (∆G), especially near the depletion region.
- A gradient in ∆G across this interface enhances hydrogen adsorption at CuO and desorption at CuWO₄, making the system more favourable for HER.
- This showcases "negative cooperativity", where increased proton binding at one site reduces affinity at other sites, facilitating proton desorption, a key step in alkaline hydrogen production.
What is Green Hydrogen?
- Green Hydrogen is produced through the electrolysis of water using renewable energy sources like solar, wind, or hydropower, releasing no greenhouse gases.
- It is a clean, sustainable, and flexible energy carrier, with water vapour as its only by-product.
- Unlike grey hydrogen (from fossil fuels), green hydrogen contributes to zero carbon emissions.
Green Hydrogen Production Methods
- Alkaline Electrolysis: Mature, low-cost method using KOH/NaOH; needs nickel/platinum
- Proton Exchange Membrane (PEM) Electrolysis: High efficiency, fast, but expensive due to precious metal catalysts.
- Solid Oxide Electrolysis (SOEC): Works at 700–1000°C, enables co-electrolysis of H₂O and CO₂, but involves complex materials and high costs.