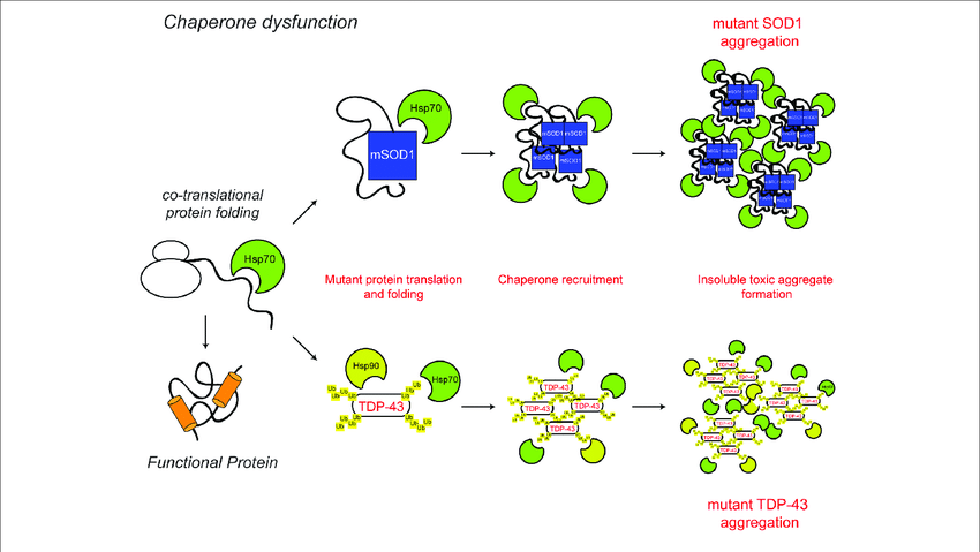
About Chaperones:
The Manjeera wildlife sanctuary in Te...
A two-story school collapsed recently...
Improved testing has led to the detec...
A team of scientists from the Nationa...
Flood situation have emerged in Bihar...
Google Fi wireless has introduced a s...
The scientists at the Indian Veterina...
A team at the S.N. Bose National Cent...
Researchers from Shenyang Agricultura...
Recently, NITI Aayog has announced th...