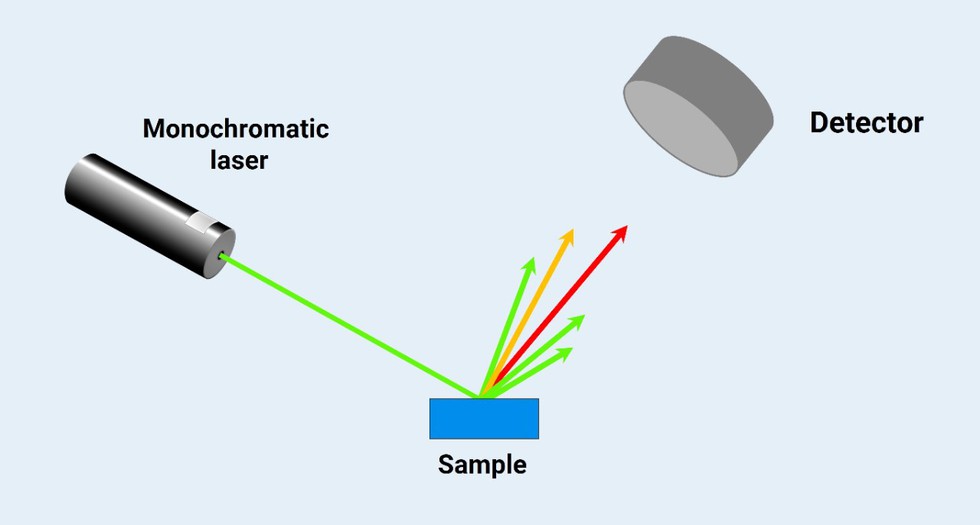
About Raman Spectroscopy:
- It is an analytical technique where scattered light is used to measure the vibrational energy modes of a sample.
- It involves illuminating a substance with a laser and analyzing the light that is scattered off the surface of the substance. It is based on the interaction of light with the chemical bonds within a material.
- It can provide both chemical and structural information, as well as the identification of substances through their characteristic Raman ‘fingerprint’.
- Raman spectroscopy extracts this information through the detection of Raman scattering from the sample.
- In 1928, Sir C.V. Raman introduced the "Raman effect," for which he was given the Nobel Prize in Physics in 1930.
- What is the Raman Effect?
- It is a change in the wavelength of light that occurs when a light beam is deflected by molecules.
- When a beam of light traverses a dust-free, transparent sample of a chemical compound, most of the scattered light is at the same wavelength (or color) as the laser source and does not provide useful information; this is called Rayleigh Scatter.
- However, a small amount of light (typically 0.0000001%) is scattered at different wavelengths (or colors), which depend on the chemical structure of the analyte; this is called Raman Scatter.