In News:
- Recently, the Drug Controller General of India (DCGI) has approved the two-dose mRNA vaccine - country’s first homegrown mRNA Covid-19 vaccine, for emergency use for the age group 18 and above.
- The vaccine - GEMCOVAC-19, developed at Pune’s Gennova Biopharmaceuticals, is stable for storage at 2-8 degree C.
What’s in today’s article:
- What are mRNA vaccines and how do they work?
- Drug Regulation in India (The Drugs and Cosmetics Act, 1940 and Rules 1945, CDSCO, DCGI)
- News Summary
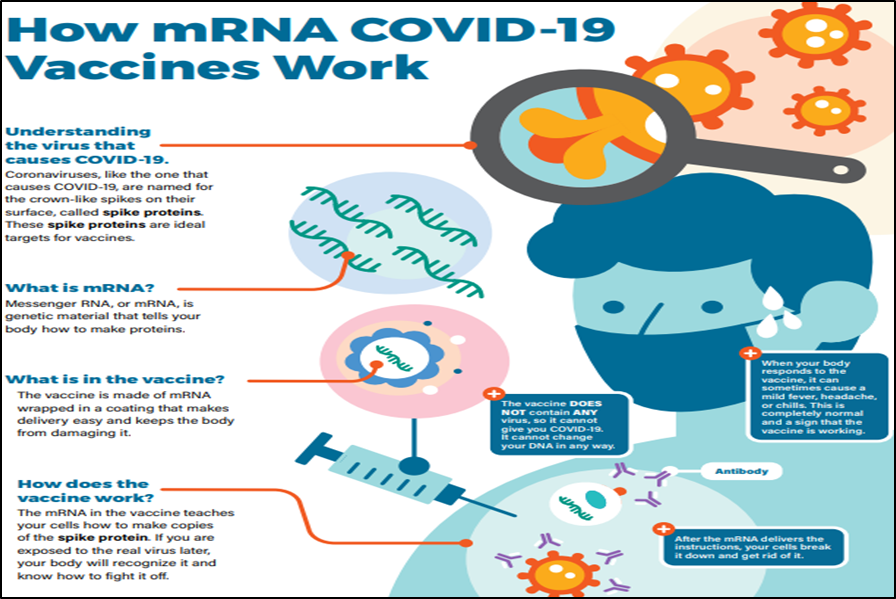
What are mRNA vaccines and how do they work?
About vaccines:
- Vaccines help prevent infection by preparing the body to fight foreign invaders (such as bacteria, viruses, or other pathogens).
- All vaccines introduce into the body a harmless piece of a particular bacteria or virus, triggering an immune response.
- Most vaccines contain a weakened or dead bacteria or virus.
- However, scientists have developed a new type of vaccine that uses a molecule called messenger ribonucleic acid (mRNA) rather than part of an actual bacteria or virus.
Messenger RNA (mRNA)
- It is a type of RNA that is necessary for protein production.
- In cells, mRNA uses the information in genes to create a blueprint for making proteins.
- Once cells finish making a protein, they quickly break down the mRNA. mRNA from vaccines does not enter the nucleus and does not alter DNA.
Significance of mRNA vaccine:
- Individuals who get an mRNA vaccine are not exposed to the virus, nor can they become infected by the vaccine.
Drug Regulation in India:
- The Drugs and Cosmetics Act, 1940 and Rules 1945: These have entrusted various responsibilities to central and state regulators for regulation of drugs and cosmetics.
- National Regulatory Authority (NRA):
- The Central Drugs Standard Control Organisation (CDSCO) under the Ministry of Health & Family Welfare is the National Regulatory Authority (NRA) of India.
- Under the Drugs and Cosmetics Act, CDSCO is responsible for -
- Approval of Drugs.
- Conduct of Clinical Trials.
- Laying down the standards for Drugs.
- Control over the quality of imported Drugs in the country.
- Coordination of the activities of State Drug Control Organizations.
- Further CDSCO along with state regulators, is jointly responsible for grant of licences of certain specialised categories of critical Drugs such as vaccine and sera, etc.
- Drugs Controller General of India (DCGI):
- DCGI is the head of department of the CDSCO. It is responsible for approval of licences of specified categories of drugs such as blood and blood products, IV fluids, vaccines and sera in India.
- DCGI also sets standards for manufacturing, sales, import, and distribution of drugs in India.
News Summary:
- Gennova already has a CDSCO licence to manufacture and sell the vaccine and has produced 70 lakh doses at risk, but now that it has received the Emergency Use Authorization (EUA), it can ship the material as soon as all formalities are completed.
- mRNA-based vaccines must be stored and distributed at extremely low temperatures.
- Because India already has a cold supply chain infrastructure capable of handling refrigeration conditions, the novel mRNA vaccine candidate, GEMCOVAC-19, which is stable at 2-8°C, can be distributed across the country using the existing refrigeration supply chain.
- The vaccine will be available to adults over the age of 18 and the two doses will be administered intramuscularly 28 days apart.









